Directed evolution improves the catalytic efficiency of TEV protease
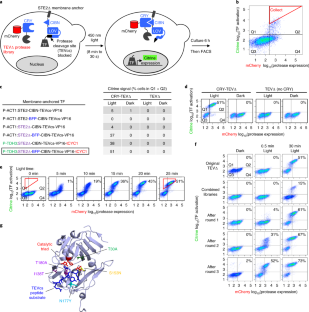
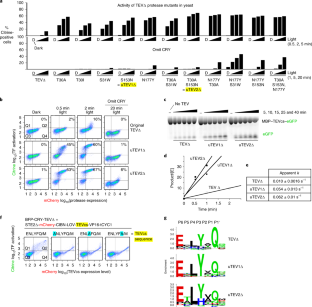
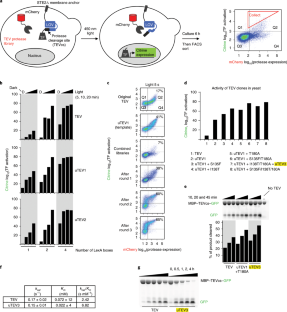
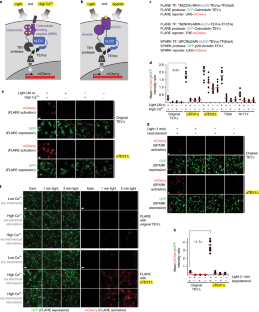
Tobacco etch virus protease (TEV) is one of the most widely used proteases in biotechnology because of its exquisite sequence specificity. A limitation, however, is its slow catalytic rate. We developed a generalizable yeast-based platform for directed evolution of protease catalytic properties. Protease activity is read out via proteolytic release of a membrane-anchored transcription factor, and we temporally regulate access to TEV’s cleavage substrate using a photosensory LOV domain. By gradually decreasing light exposure time, we enriched faster variants of TEV over multiple rounds of selection. Our TEV-S153N mutant (uTEV1Δ), when incorporated into the calcium integrator FLARE, improved the signal/background ratio by 27-fold, and enabled recording of neuronal activity in culture with 60-s temporal resolution. Given the widespread use of TEV in biotechnology, both our evolved TEV mutants and the directed-evolution platform used to generate them could be beneficial across a wide range of applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
265,23 € per year
only 22,10 € per issue
Buy this article
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
On the cutting edge: protease-based methods for sensing and controlling cell biology
Article 13 July 2020
Robust and flexible platform for directed evolution of yeast genetic switches
Article Open access 23 March 2021
Engineering orthogonal signalling pathways reveals the sparse occupancy of sequence space
Article 23 October 2019
Data availability
Additional data beyond that provided in the Figures and Supplementary Information are available from the corresponding author upon request.
Change history
References
- Liu, Q. et al. A photoactivatable botulinum neurotoxin for inducible control of neurotransmission. Neuron101, 863–875 (2019).
- Smart, A. D. et al. Engineering a light-activated caspase-3 for precise ablation of neurons in vivo. Proc. Natl Acad. Sci. USA114, E8174–E8183 (2017). ArticleCASGoogle Scholar
- Lin, M. Z., Glenn, J. S. & Tsien, R. Y. A drug-controllable tag for visualizing newly synthesized proteins in cells and whole animals. Proc. Natl Acad. Sci. USA105, 7744–7749 (2008). ArticleCASGoogle Scholar
- Schuster, B. S. et al. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. Nat. Commun.9, 2985 (2018). ArticleGoogle Scholar
- Gao, X. J., Chong, L. S., Kim, M. S. & Elowitz, M. B. Programmable protein circuits in living cells. Science361, 1252–1258 (2018). ArticleCASGoogle Scholar
- Fink, T. et al. Design of fast proteolysis-based signaling and logic circuits in mammalian cells. Nat. Chem. Biol.15, 115–122 (2019). ArticleCASGoogle Scholar
- Copeland, M. F., Politz, M. C., Johnson, C. B., Markley, A. L. & Pfleger, B. F. A transcription activator–like effector (TALE) induction system mediated by proteolysis. Nat. Chem. Biol.12, 254–260 (2016). ArticleCASGoogle Scholar
- Wang, W. et al. A light- and calcium-gated transcription factor for imaging and manipulating activated neurons. Nat. Biotechnol.35, 864–871 (2017). ArticleCASGoogle Scholar
- Lee, D., Hyun, J. H., Jung, K., Hannan, P. & Kwon, H.-B. A calcium- and light-gated switch to induce gene expression in activated neurons. Nat. Biotechnol.35, 858–863 (2017). ArticleCASGoogle Scholar
- Barnea, G. et al. The genetic design of signaling cascades to record receptor activation. Proc. Natl Acad. Sci. USA105, 64–69 (2008). ArticleCASGoogle Scholar
- Kim, M. W. et al. Time-gated detection of protein-protein interactions with transcriptional readout. eLife6, e30233 (2017). ArticleGoogle Scholar
- Kim, C. K., Cho, K. F., Kim, M. W. & Ting, A. Y. Luciferase-LOV BRET enables versatile and specific transcriptional readout of cellular protein-protein interactions. eLife8, e43826 (2019). ArticleGoogle Scholar
- Parks, T. D., Howard, E. D., Wolpert, T. J., Arp, D. J. & Dougherty, W. G. Expression and purification of a recombinant tobacco etch virus NIa proteinase: biochemical analyses of the full-length and a naturally occurring truncated proteinase form. Virology210, 194–201 (1995). ArticleCASGoogle Scholar
- Evnin, L. B., Vásquez, J. R. & Craik, C. S. Substrate specificity of trypsin investigated by using a genetic selection. Proc. Natl Acad. Sci. USA87, 6659–6663 (1990). ArticleCASGoogle Scholar
- Estell, D. A., Graycar, T. P. & Wells, J. A. Engineering an enzyme by site-directed mutagenesis to be resistant to chemical oxidation. J. Biol. Chem.260, 6518–6521 (1985). ArticleCASGoogle Scholar
- Packer, M. S., Rees, H. A. & Liu, D. R. Phage-assisted continuous evolution of proteases with altered substrate specificity. Nat. Commun.8, 956 (2017). ArticleGoogle Scholar
- Yi, L. et al. Engineering of TEV protease variants by yeast ER sequestration screening (YESS) of combinatorial libraries. Proc. Natl Acad. Sci. USA110, 7229–7234 (2013). ArticleCASGoogle Scholar
- Lam, S. S. et al. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods12, 51–54 (2015). ArticleCASGoogle Scholar
- Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol.36, 880–887 (2018). ArticleCASGoogle Scholar
- Martell, J. D. et al. A split horseradish peroxidase for the detection of intercellular protein–protein interactions and sensitive visualization of synapses. Nat. Biotechnol.34, 774–780 (2016). ArticleCASGoogle Scholar
- Han, Y. et al. Directed evolution of split APEX2 peroxidase. ACS Chem. Biol. 14, 619–635 (2019).
- Kennedy, M. J. et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods7, 973–975 (2010). ArticleCASGoogle Scholar
- Kapust, R. B. et al. Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng.14, 993–1000 (2001). ArticleCASGoogle Scholar
- Phan, J. et al. Structural basis for the substrate specificity of tobacco etch virus protease. J. Biol. Chem.277, 50564–50572 (2002). ArticleCASGoogle Scholar
- Raran-Kurussi, S., Tözsér, J., Cherry, S., Tropea, J. E. & Waugh, D. S. Differential temperature dependence of tobacco etch virus and rhinovirus 3C protease. s. Anal. Biochem.436, 142–144 (2013). ArticleCASGoogle Scholar
- Kostallas, G., Löfdahl, P.-Å. & Samuelson, P. Substrate profiling of tobacco etch virus protease using a novel fluorescence-assisted whole-cell assay. PLoS One6, e16136 (2011). ArticleCASGoogle Scholar
- Li, Q. et al. Profiling protease specificity: combining yeast ER sequestration screening (YESS) with next generation sequencing. ACS Chem. Biol.12, 510–518 (2017). ArticleCASGoogle Scholar
- Kapust, R. B., Tözsér, J., Copeland, T. D. & Waugh, D. S. The P1′ specificity of tobacco etch virus protease. Biochem. Biophys. Res. Commun.294, 949–955 (2002). ArticleCASGoogle Scholar
- Thomsen, M. C. F. & Nielsen, M. Seq2Logo: a method for construction and visualization of amino acid binding motifs and sequence profiles including sequence weighting, pseudo counts and two-sided representation of amino acid enrichment and depletion. Nucleic Acids Res40, W281–W287 (2012). ArticleCASGoogle Scholar
- Cabrita, L. D. et al. Enhancing the stability and solubility of TEV protease using in silico design. Protein Sci.16, 2360–2367 (2007). ArticleCASGoogle Scholar
- Sente, A. et al. Molecular mechanism of modulating arrestin conformation by GPCR phosphorylation. Nat. Struct. Mol. Biol.25, 538–545 (2018). ArticleCASGoogle Scholar
- Martell, J. D. et al. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat. Biotechnol.30, 1143–1148 (2012). ArticleCASGoogle Scholar
- Strickland, D. et al. Rationally improving LOV domain–based photoswitches. Nat. Methods7, 623–626 (2010). ArticleCASGoogle Scholar
- Turk, B. E., Huang, L. L., Piro, E. T. & Cantley, L. C. Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat. Biotechnol.19, 661–667 (2001). ArticleCASGoogle Scholar
- Wiita, A. P., Seaman, J. E. & Wells, J. A. Global analysis of cellular proteolysis by selective enzymatic labeling of protein N-termini. Methods Enzymol.544, 327–358 (2014). ArticleCASGoogle Scholar
- Kim, J. H. et al. High cleavage efficiency of a 2 A peptide derived from porcine Teschovirus-1 in human cell lines, zebrafish and mice. PLoS One6, e18556 (2011). ArticleCASGoogle Scholar
- Ottoz, D. S. M., Rudolf, F. & Stelling, J. Inducible, tightly regulated and growth condition-independent transcription factor in saccharomyces cerevisiae. Nucleic Acids Res.42, e130–e130 (2014). ArticleGoogle Scholar
- Peng, B., Williams, T. C., Henry, M., Nielsen, L. K. & Vickers, C. E. Controlling heterologous gene expression in yeast cell factories on different carbon substrates and across the diauxic shift: a comparison of yeast promoter activities. Microb. Cell Fact.14, 91 (2015). ArticleGoogle Scholar
- Swiech, L. et al. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat. Biotechnol.33, 102–106 (2015). ArticleCASGoogle Scholar
- Loh, K. H. et al. Proteomic analysis of unbounded cellular compartments: synaptic clefts. Cell166, 1295–1307.e21 (2016). ArticleCASGoogle Scholar
- Tropea, J. E., Cherry, S. & Waugh, D. S. Expression and purification of soluble His6-tagged TEV protease. Methods Mol. Biol.498, 297–307 (2009). ArticleCASGoogle Scholar
Acknowledgements
We are grateful to Stanford, the Chan Zuckerberg Biohub, the Beckman Technology Development Seed Grant and NIH (R01 MH119353) for support of this work. FACS was performed at the MIT Koch Institute Flow Cytometry Core and at the Stanford Shared FACS Facility. W. Wang (University of Michigan) provided plasmids and advice. L. Ning (Stanford University) provided rat brain tissue. A. G. Johnson (Stanford) gave advice on TEV expression, and N. Samiylenko helped reproduce some experiments. B. Babin, J. Yim and M. Bogyo (Stanford University) provided access to their HPLC. G. Liu (MIT) built the LED box used for blue light irradiation of cells. M. Djuristic (Stanford University) assisted with electrical stimulation of neurons. M.I.S. was supported by an EMBO long-term post-doctoral fellowship (ALTF 1022-2015).
Author information
Authors and Affiliations
- Departments of Genetics, Biology and Chemistry, Stanford University, Stanford, CA, USA Mateo I. Sanchez & Alice Y. Ting
- Chan Zuckerberg Biohub, San Francisco, CA, USA Mateo I. Sanchez & Alice Y. Ting
- Mateo I. Sanchez








